Teaching kids about acids and bases doesn’t need to be met with groans and complaints. This is a science lesson that can easily be made fun and exciting with a few simple hands-on activities about acids & bases.
Here we’ve provided an intro to acids and bases for kids, as well as five fun projects to reinforce the lesson.
Table of Contents
What are Acids and Bases?
Although “acid” might sound like something dangerous and “base” could remind you of a ball field, acids and bases are common substances found right around your house!
Juices like orange, lemons, and tomato are acids, for instance. They often taste a bit sour.
Baking soda, soaps, and chalk (that can mark the bases on a ball field) are all considered bases. When mixed with water, they feel slimy or slippery. Certain acids and bases are, in fact, dangerous. Battery acid can eat right through the fabric. Ammonia is a base that can be harmful to smell closely, especially for people with asthma.
—–Download our Free 25 STEM Activities eBook.—–
What’s the Difference Between Acids and Bases?
All these substances have ions. Ions are tiny molecules or atoms of distinct types.
Acids tend to have many hydrogen ions, giving a tangy taste to foods.
On the other hand, bases hold many hydroxide ions, which can taste bitter in things that are edible. Acids and bases appear in nature and right in our bodies. Our stomachs have acids to help digest food. The various parts of plants may be acids or bases.
How Can We Measure Acidity or Baseness?
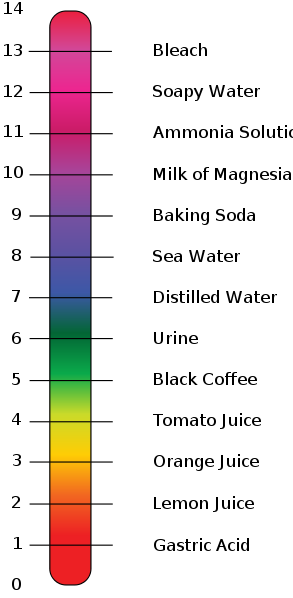
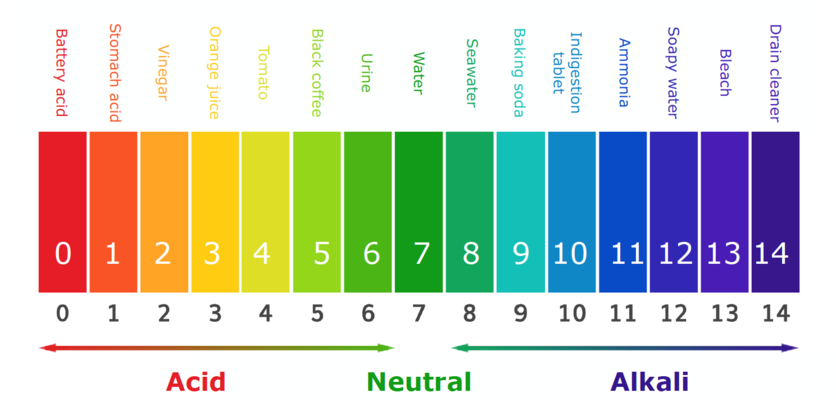
Look at the picture, above, of the pH scale, which we use to graphically compare acidity and baseness. The part of the scale from zero up to 7 stands for acids, 7 is neutral, and everything above 7 stands for bases.
How can we measure substances to decide where they fall on the pH scale? Certain things we can taste, to help make a good guess where they might fall on the scale. Other substances we can’t sample in that way.
Instead, we need to use something called an indicator. Indicators are materials from nature that turn a certain color to show acidity or baseness. For example, purple litmus paper is made from special plants and will turn red when it contacts acids and turns blue when it touches bases.
Here are the spots where select common substances fall on the pH scale:
Now that you know foundation facts about bases and acids, let’s try a few fun activities!
6 Fun Projects to Teach Kids About Acids & Bases
1. Red Cabbage as an Indicator
You can make something like litmus paper at home with red cabbage and use it to test different acids and bases.
Slice and cook the cabbage on top of the stove in simmering water. After it’s cooled, strain and keep the colored juice. You can also save the cabbage and add it to your favorite stew for dinner.
Dip several white index cards into the cooled cabbage juice and let them dry. After they’re dry you can cut into strips to test various substances. Dip them in things like pickle juice and milk, guessing ahead of time whether they will turn the paper red for acid or blue for a base.
Pour the cabbage juice into several clear glasses. Add various liquids to see what color the juice will become. You might try cola in one and baking soda in another to see what color the water will magically turn. Keep track of your results.
2. Edible Chemical Reaction
When acids and bases meet, you might see interesting reactions. Some of these combinations aren’t safe to eat. However, here’s a combination that’s edible and tasty.
That frozen treat called “sherbet” contains both an acid and a base. Try making your own version and see if you can feel the reaction in your mouth!
In a dry bowl or in a sealable sandwich bag combine the following ingredients:
- 1/4 teaspoon baking soda
- 1/2 teaspoon citric acid crystals (from supplement/vitamin stores)
- 1/2 teaspoon sweetened drink crystals (like Kool-Ade)
- ·1 teaspoon powdered sugar
Stir, if using the bowl, or seal and shake if using a bag. After it’s smooth, try eating a little at a time. What happens in your mouth? Discuss which of the ingredients you think are bases and which are acids.
3. Clean Those Pennies

Find several tarnished, dirty-looking pennies. In three or four clear glasses make solutions, or mixtures, combining water with acids and with bases, such as lemon juice, cola, baking soda, or soap.
Which mixtures do you predict will clean and shine the pennies the best, when the coins remain in the water for a while? Write down your predictions and compare them to your results.
4. Make an Egg Shell Disappear

Carefully lower a raw egg into a bowl or glass of white vinegar. Guess what you think might happen. Then let the egg rest in the white vinegar overnight.
After about 24 hours, you should find that the acid in the vinegar dissolves the shell (calcium carbonate). You’ve now made your very own “rubber egg!”
(NOTE: You wouldn’t want to eat this because of possible bacteria still contained in the egg.)
5. Combining Acids and Bases
What do you think will happen when acids and bases are combined? You might find they can neutralize each other or move closer to the neutral range on the pH scale.
Test this by placing spooned baking soda into a glass of vinegar. Then test the solution with litmus paper or with your homemade paper from the cabbage experiment.
What are your results? What did you observe when the baking soda first met up with the vinegar?
Wrapping Up
What are your favorite projects for teaching kids about acids and bases? Let us know in the comments!
Plus, don’t forget to download our free scientific method worksheet that kids can use while conducting their experiments on acids & bases.